OUR PATIENT RECRUITMENT COMPANY MANAGES CLINICAL TRIALS WORLDWIDE
Recruit Eligible, Diverse Patients Faster with our Trial Embrace™ Enrollment Services
Trial AMPlify is the only patient recruitment company to offer a 100% satisfaction guarantee based on proven ability to recruit patients for your study. We make it easy for you to move forward with us.
Measurable Patient Recruitment Results for Your Clinical Studies
Choosing the Right Partner for Clinical Trial Success
For drug discovery sponsors, clinical investigators, and Contract Research Organizations (CROs), selecting an effective patient recruitment agency is pivotal. Trial AMPlify sets itself apart by prioritizing patient engagement through proven digital marketing strategies tailored for clinical trials. We specialize in reaching patients and their families where they are most active. A Facebook campaign alone is not a strategy. Trial AMPlify is able to triangulate targeted Google Ad campaigns, SEO Page #1 results and Programmatic Banner Display Advertising.
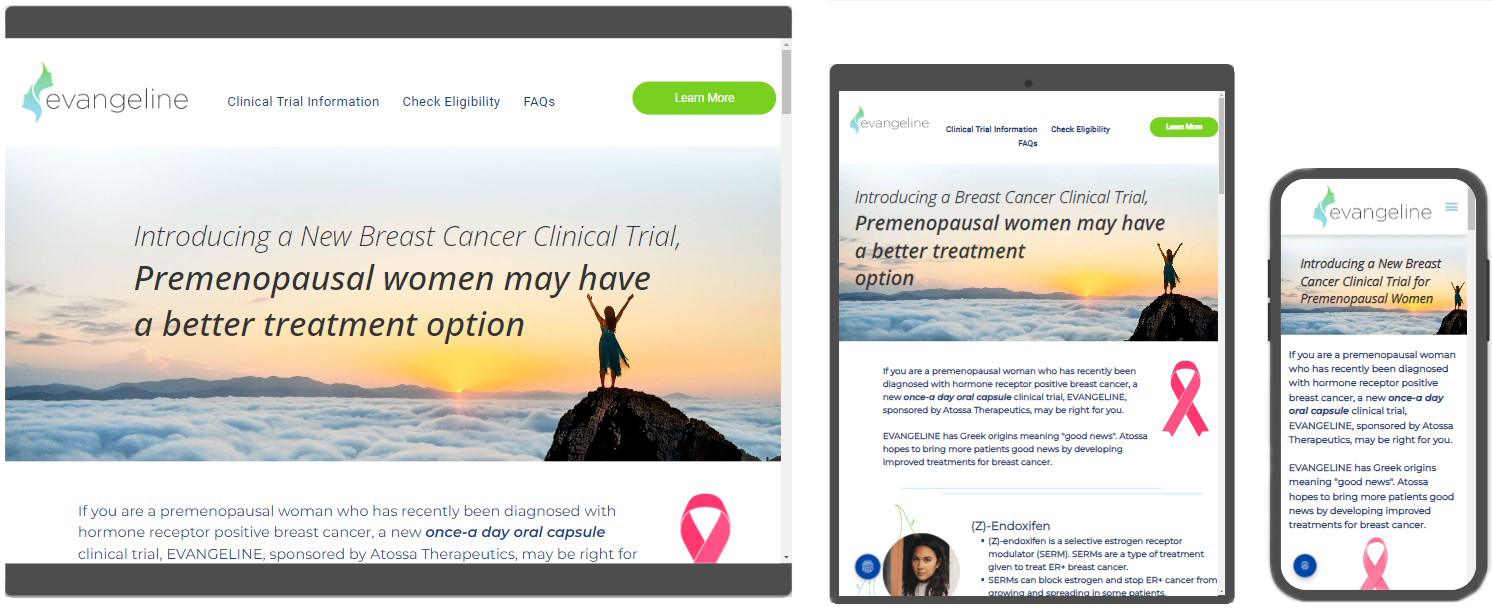
Tailored Clinical Trial Landing Pages
At Trial
AMPlify, we understand the importance of clear and accessible information regarding clinical trials. Our custom-designed clinical trial websites detail your trial protocols, eligibility requirements, site locations, and facilitating a smoother, more informative web visitor journey for potential subjects. This approach is the
# 1 best way to increase diversity within your patient pool, making the trial process transparent, and empowering patients to discover and understand trials. A dedicated clinical trial landing page fosters a higher rate of self-identification and more subjects reaching out to join the trial.
No sponsor should be using their CT.gov listing as the face of their study. Doing so is a disservice to patients and hobbles your subject enrollment.
Award-Winning Design and Compliance Standards
Our clinical trial website design team has been recognized for its innovative solutions that combine aesthetic appeal with functionality, ensuring compliance with the highest HIPAA standards for security and patient privacy. Our commitment to diversity-centric patient recruitment and our satisfaction guarantee further reinforce Trial AMPlify's position as the leader in patient recruitment and clinical trial marketing.
Contact Trial AMPlify for a VIP Consultation
Discover how Trial
AMPlify
can help you
surge patient enrollment
for your clinical trials with our cutting-edge digital marketing solutions. Our services are custom tailored to boost your trial's visibility and participant engagement. We'll show you why our life science and biopharma clients trust us to fill their study.
Contact us today to see our campaigns in action. You'll be in good company.









